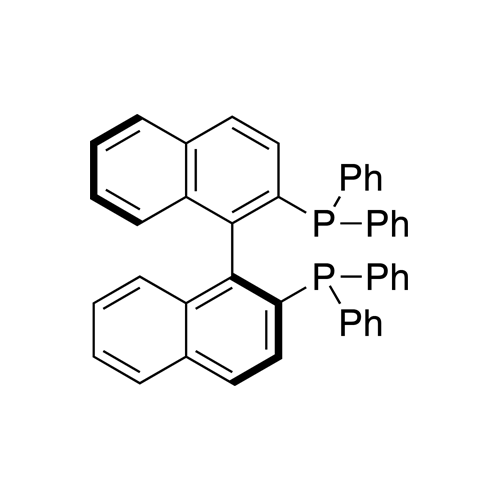
(S)-(−)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene, min 97%
Synonym: (S)-(−)-(1,1′-Binaphthalene-2,2′-diyl)bis(diphenylphosphine),
(S)-(−)-BINAP
CAS Number:76189-56-5
Molecular Formula: C44H32P2
Formula Weight:622.70
Color and Form:white to off-white pwdr.
melting point 241-242°C
Storage:Keep container tightly closed in a dry and well-ventilated place.
Handle under nitrogen, protect from moisture. Store under nitrogen. Air and moisture sensitive. Non Combustible Solids
Precautions for safe handling:
Further processing of solid materials may result in the formation of combustible dusts. Thepotential for combustible dust formation should be taken into consideration before additional processing occurs.
Provide appropriate exhaust ventilation at places where dust is formed.
Application
2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl and its rhodium and ruthenium derivatives are highly selective homogeneous catalysts used for the reduction of aryl ketones,β-keto esters,and α-amino ketones. They have also been used for asymmetric hydrogenation and hydroformylation of olefins, asymmetric Heck reactions,and asymmetric isomerizations of allyls.
Ligand used in a palladium-catalyzed, asymmetric, tandem Heck reaction-carbanion capture process leading to a synthesis of a tricyclic sesquiterpene.Also used in a ruthenium-catalyzed asymmetric hydrogenation of α,β-unsaturated acids.
Reactant involved in:
• Enantioselective and diastereoselective unpoled carbonyl allylation
• Syntehsis of organophophine oxides as anittumor agents
• SN2 halogenation of hydroxy groups
• Synthesis of BINAP complexes
• Studies of conformational flexibility of BINAP chelates
Related Categories
Asymmetric Synthesis, BINAPs, Chemical Synthesis, Chiral Catalysts, Ligands, and Reagents, Privileged Ligands and Complexes
